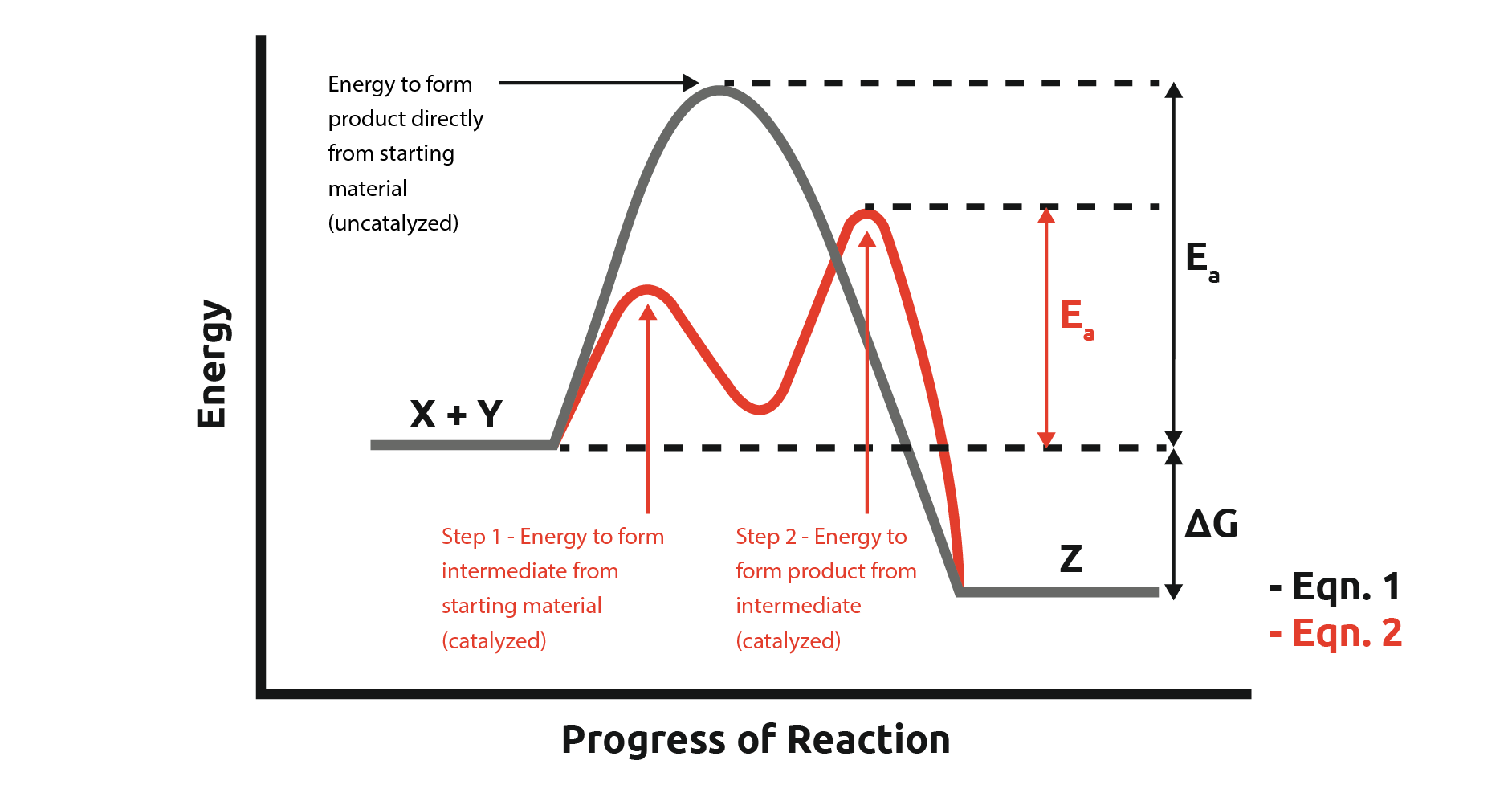
Catalyst And Intermediate Reaction . a second way to make the reaction go faster is to employ a catalyst. goes over two examples that highlight the differences between catalysts and intermediates. this chemistry video tutorial explains how to identify the intermediate and. You probably already know that a catalyst is an agent that causes a chemical reaction to go. the catalyst provides a different reaction path with a lower activation energy. A catalyst is used at the. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more.
from www.labunlimited.com
in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. goes over two examples that highlight the differences between catalysts and intermediates. this chemistry video tutorial explains how to identify the intermediate and. A catalyst is used at the. a second way to make the reaction go faster is to employ a catalyst. the catalyst provides a different reaction path with a lower activation energy. You probably already know that a catalyst is an agent that causes a chemical reaction to go.
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited
Catalyst And Intermediate Reaction You probably already know that a catalyst is an agent that causes a chemical reaction to go. You probably already know that a catalyst is an agent that causes a chemical reaction to go. the catalyst provides a different reaction path with a lower activation energy. goes over two examples that highlight the differences between catalysts and intermediates. A catalyst is used at the. this chemistry video tutorial explains how to identify the intermediate and. a second way to make the reaction go faster is to employ a catalyst. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst And Intermediate Reaction the catalyst provides a different reaction path with a lower activation energy. this chemistry video tutorial explains how to identify the intermediate and. a second way to make the reaction go faster is to employ a catalyst. A catalyst is used at the. in general, catalytic action is a chemical reaction between the catalyst and a. Catalyst And Intermediate Reaction.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst And Intermediate Reaction goes over two examples that highlight the differences between catalysts and intermediates. the catalyst provides a different reaction path with a lower activation energy. a second way to make the reaction go faster is to employ a catalyst. You probably already know that a catalyst is an agent that causes a chemical reaction to go. A catalyst. Catalyst And Intermediate Reaction.
From slideplayer.com
Reaction Mechanism The reaction mechanism is the series of elementary Catalyst And Intermediate Reaction You probably already know that a catalyst is an agent that causes a chemical reaction to go. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. A catalyst is used at the. goes over two examples that highlight the differences between catalysts and intermediates.. Catalyst And Intermediate Reaction.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Catalyst And Intermediate Reaction goes over two examples that highlight the differences between catalysts and intermediates. this chemistry video tutorial explains how to identify the intermediate and. A catalyst is used at the. the catalyst provides a different reaction path with a lower activation energy. a second way to make the reaction go faster is to employ a catalyst. . Catalyst And Intermediate Reaction.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst And Intermediate Reaction a second way to make the reaction go faster is to employ a catalyst. A catalyst is used at the. this chemistry video tutorial explains how to identify the intermediate and. You probably already know that a catalyst is an agent that causes a chemical reaction to go. the catalyst provides a different reaction path with a. Catalyst And Intermediate Reaction.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalyst And Intermediate Reaction in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. the catalyst provides a different reaction path with a lower activation energy. this chemistry video tutorial explains how to identify the intermediate and. A catalyst is used at the. You probably already know that. Catalyst And Intermediate Reaction.
From www.slideserve.com
PPT Reaction Mechanism PowerPoint Presentation, free download ID Catalyst And Intermediate Reaction A catalyst is used at the. a second way to make the reaction go faster is to employ a catalyst. You probably already know that a catalyst is an agent that causes a chemical reaction to go. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to. Catalyst And Intermediate Reaction.
From philschatz.com
Catalysis · Chemistry Catalyst And Intermediate Reaction this chemistry video tutorial explains how to identify the intermediate and. You probably already know that a catalyst is an agent that causes a chemical reaction to go. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. a second way to make the. Catalyst And Intermediate Reaction.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst And Intermediate Reaction this chemistry video tutorial explains how to identify the intermediate and. A catalyst is used at the. the catalyst provides a different reaction path with a lower activation energy. a second way to make the reaction go faster is to employ a catalyst. in general, catalytic action is a chemical reaction between the catalyst and a. Catalyst And Intermediate Reaction.
From slideplayer.com
Reaction Mechanisms ppt download Catalyst And Intermediate Reaction in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. You probably already know that a catalyst is an agent that causes a chemical reaction to go. the catalyst provides a different reaction path with a lower activation energy. A catalyst is used at the.. Catalyst And Intermediate Reaction.
From www.slideserve.com
PPT Section 14.6 Reaction Mechanisms and Catalysis PowerPoint Catalyst And Intermediate Reaction the catalyst provides a different reaction path with a lower activation energy. goes over two examples that highlight the differences between catalysts and intermediates. this chemistry video tutorial explains how to identify the intermediate and. a second way to make the reaction go faster is to employ a catalyst. A catalyst is used at the. . Catalyst And Intermediate Reaction.
From quizdbcornwallis.z21.web.core.windows.net
What Is A Reaction Intermediate Catalyst And Intermediate Reaction the catalyst provides a different reaction path with a lower activation energy. A catalyst is used at the. a second way to make the reaction go faster is to employ a catalyst. goes over two examples that highlight the differences between catalysts and intermediates. this chemistry video tutorial explains how to identify the intermediate and. . Catalyst And Intermediate Reaction.
From lavelle.chem.ucla.edu
Intermediate vs. Catalyst CHEMISTRY COMMUNITY Catalyst And Intermediate Reaction the catalyst provides a different reaction path with a lower activation energy. this chemistry video tutorial explains how to identify the intermediate and. goes over two examples that highlight the differences between catalysts and intermediates. A catalyst is used at the. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming. Catalyst And Intermediate Reaction.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst And Intermediate Reaction this chemistry video tutorial explains how to identify the intermediate and. a second way to make the reaction go faster is to employ a catalyst. the catalyst provides a different reaction path with a lower activation energy. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. Catalyst And Intermediate Reaction.
From www.youtube.com
Reaction Intermediates and Catalyst Examples YouTube Catalyst And Intermediate Reaction goes over two examples that highlight the differences between catalysts and intermediates. this chemistry video tutorial explains how to identify the intermediate and. the catalyst provides a different reaction path with a lower activation energy. A catalyst is used at the. a second way to make the reaction go faster is to employ a catalyst. . Catalyst And Intermediate Reaction.
From 2012books.lardbucket.org
Catalysis Catalyst And Intermediate Reaction in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. goes over two examples that highlight the differences between catalysts and intermediates. the catalyst provides a different reaction path with a lower activation energy. this chemistry video tutorial explains how to identify the. Catalyst And Intermediate Reaction.
From chemistnotes.com
Reaction Intermediates, Example, and Types Chemistry Notes Catalyst And Intermediate Reaction goes over two examples that highlight the differences between catalysts and intermediates. A catalyst is used at the. this chemistry video tutorial explains how to identify the intermediate and. a second way to make the reaction go faster is to employ a catalyst. You probably already know that a catalyst is an agent that causes a chemical. Catalyst And Intermediate Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst And Intermediate Reaction goes over two examples that highlight the differences between catalysts and intermediates. the catalyst provides a different reaction path with a lower activation energy. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more. A catalyst is used at the. a second way. Catalyst And Intermediate Reaction.